- Mobile:+86-21-61659322
- Email:export@sun-chem.com
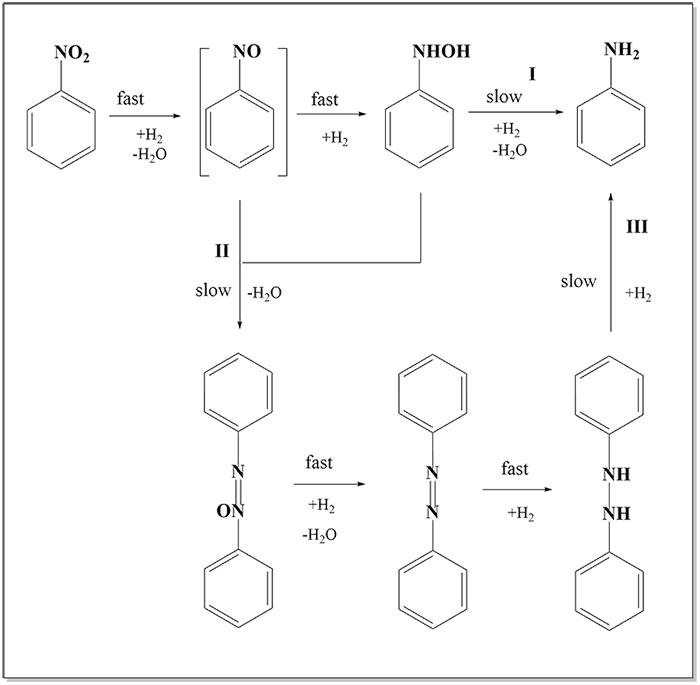
Nitro Hydrogenation
In the hydrogenation process of aromatic nitro, the hydrogenation activity of intermediate — hydroxylamine is higher (I), ensuring high selectivity and better inhibiting side reaction (II). The by-product azo will also be hydrogenated to amino by highly active catalyst (III). The reaction mechanism is shown in the following figure:
| Product | Metal content/type | Carrier | Shape | Size | Applications |
RaneCAT-1000  | ≥90%Ni | None | Powder | D50:23-33um |
|
RaneCAT-1100  | ≥90%Ni | None | Powder | D50:30-45um |
|
RaneCAT-1300  | ≥90%Ni | None | Powder | D50:50-70um |
|
| Pd | Al2O3 | Pellet | 2-3mm |
|
| Pt | Al2O3 | Pellet | 2-3mm |
|
| Ru | Al2O3 | Pellet | 2-3mm |
|



