- Mobile:+86-21-61659322
- Email:export@sun-chem.com
Alcohols Dehydrogenation
Dehydrogenation is the reaction of organic compound dehydrogenation under high temperature and catalyst, and is the reverse reaction of hydrogenation. When the dehydrogenation reactant contacts with the catalyst, hydrogen atoms are activated and dissociated. At the same time, the reactant binds with the catalyst, and the free hydrogen acts on the adjacent hydrogen atoms to get them dissociated and form H2 molecules and double bonds. Then the dehydrogenation reaction is completed.
Dehydrogenation mechanism is as follows:
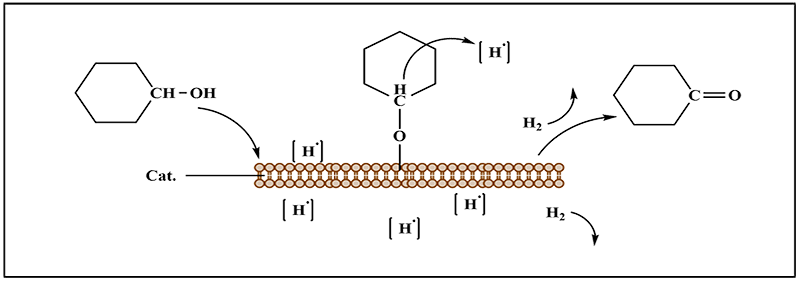
SUNCHEM is providing dehydrogenation catalysts for liquid phase and gas phase application.
Product | Metal content/type | Carrier | Shape | Size | Applications |
95% Cu | None | Powder | 35μm |
| |
Cu | SiO2 | Tablet | 5*3mm |
| |
Cu | SiO2 | Tablet | 5*3mm |
|



